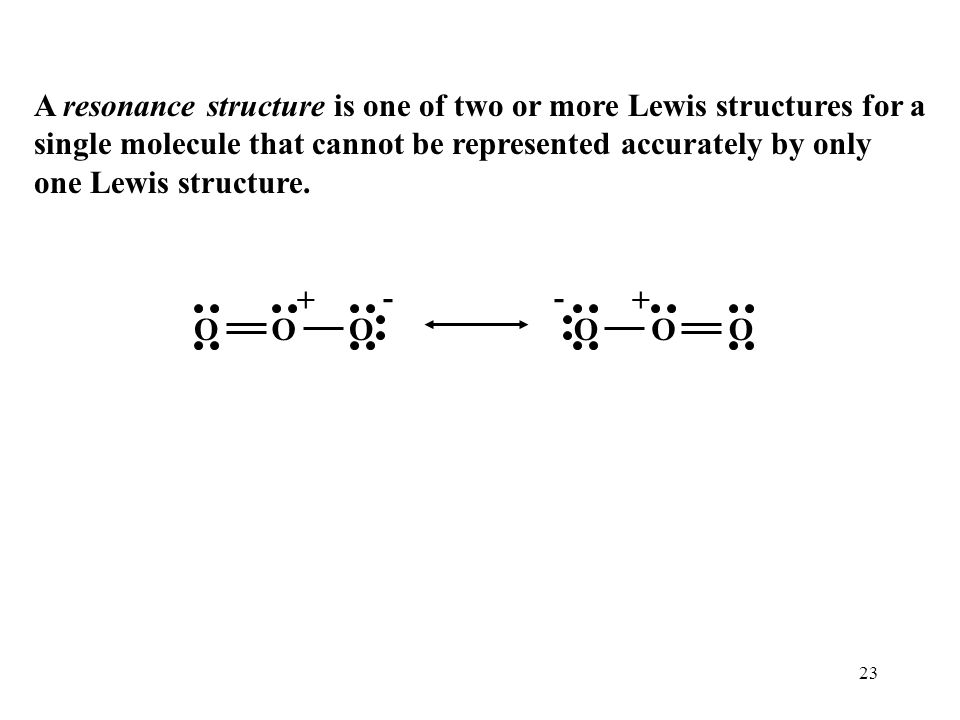
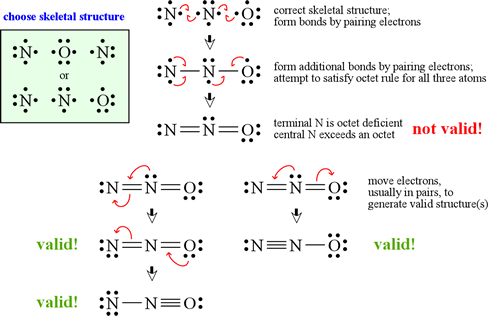
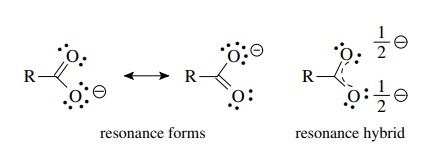
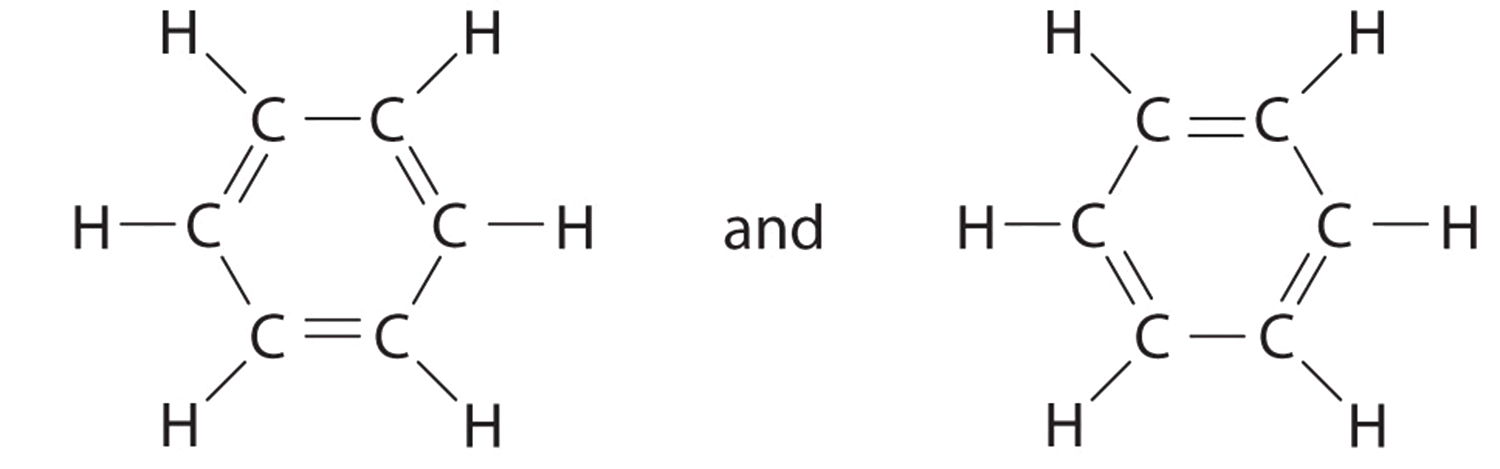Question 44 Explain why CO 32- ion cannot be represented by a single Lewis structure. How can it be best represented?
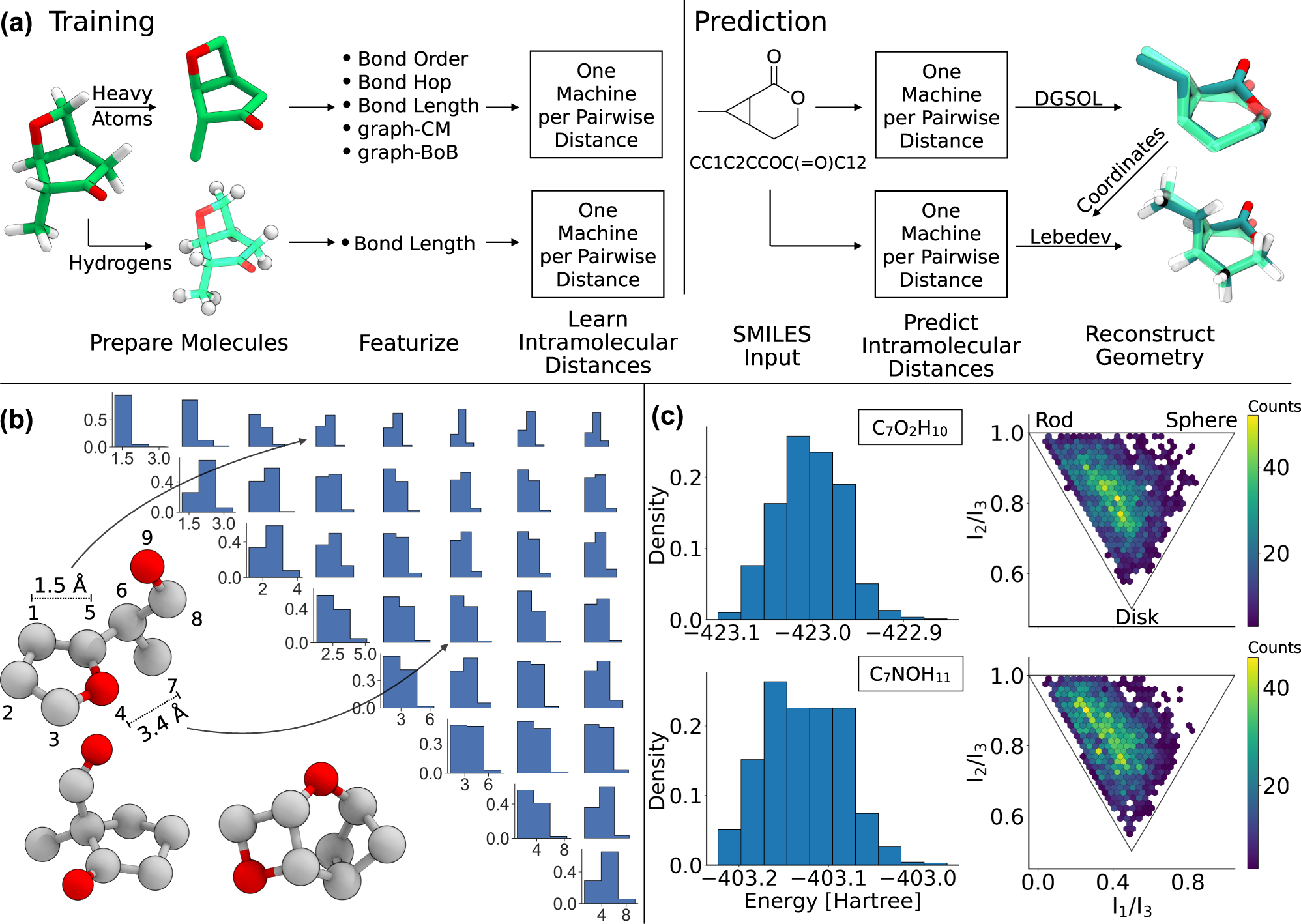
Machine learning based energy-free structure predictions of molecules, transition states, and solids | Nature Communications

Basic Concepts of Chemical Bonding Chapter 8. Three Types of Chemical Bonds Ionic bond Ionic bond –Transfer of electrons –Between metal and nonmetal ions. - ppt download

Explain why CO_{3}^{2-} ion cannot be represented by a single Lewis structure. How can it be best represented?